Body Warmer
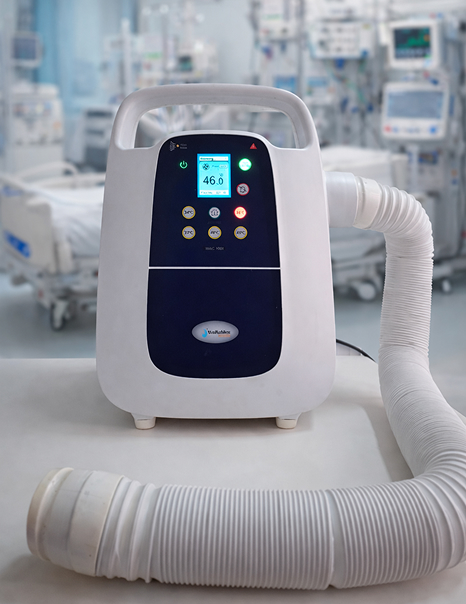
An early-stage medical device founder with a vision for a clinical Body Warmer device approached NPMedTech after repeated development failures with a previous vendor.
The goal was to rescue the project and deliver a safe, compliant, and manufacturable medical device.
Overview
A medical device founder approached NPMedTech with a stalled Body Warmer device project after 18 months of unsuccessful development with another vendor. The product remained non-functional, unsafe, and far from certification. The objective was to rescue the project and deliver a certified, production-ready medical device.
The Challenge
The project was hindered by a non-functional and unsafe prototype, unstable firmware, and non-compliant hardware. There was no design documentation or clear certification pathway, and development had completely stalled, placing the entire project at risk of shutdown.
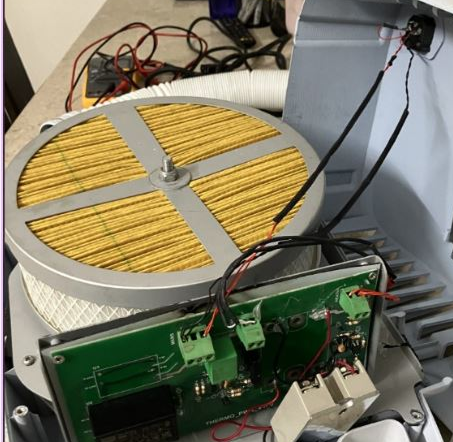

Our Solution
NPMedTech rebuilt the hardware, electronics, and embedded firmware from the ground up and redesigned the user interface to support clinical usability. Comprehensive safety controls, diagnostics, and fail-safe mechanisms were implemented, while the complete IEC 60601 certification and testing process was managed. The design was also prepared for manufacturing and future scale.
The Result
Within nine months, a fully functional and certified Body Warmer medical device was delivered. The product is now deployed in hospitals and supported by complete production-ready documentation.
